Bacterial biofilm destruction by size/surface charge-adaptive micelles†
Abstract
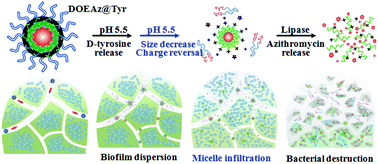
Biofilms formed by pathogenic bacteria are one of the most important reasons for multidrug resistance. One of the major limitations in the biofilm treatment is the existence of intensive matrices, which greatly block the diffusion of antimicrobial agents. In the current study, we designed poly(aspartamide)-derived micelles self-assembled from cationic copolymers with azithromycin-conjugated and pH-sensitive copolymers, followed by loading cis-aconityl-D-tyrosine (CA-Tyr) via electrostatic interactions. In response to the acidic microenvironment of the biofilm matrix, the hydrophilic transition of the pH-sensitive copolymers and the removal of CA-Tyr led to a sharp decrease in micelle size from 107 nm to 54 nm and a rapid shift in their zeta potential from −11.7 mV to +26.4 mV, which facilitated the penetration of the micelles into biofilms. The acid-labile release of D-tyrosine disintegrated the biofilm matrix, and the lipase-triggered release of azithromycin eradicated the bacteria in the biofilms. An in vitro test was performed on pre-established P. aeruginosa biofilms in microwells, while biofilms grown on catheters were surgically implanted in rats for in vivo evaluation. The results demonstrated the capabilities of the size/surface charge-adaptive micelles in the intensive infiltration in the biofilm matrix and spatiotemporal release of biofilm dispersion and antibacterial agents for the comprehensive treatment of biofilm-relevant infections.


 Please wait while we load your content...
Please wait while we load your content...