Hydrolysis of trialkoxysilanes catalysed by the fluoride anion. Nucleophilic vs. basic catalysis†
Abstract
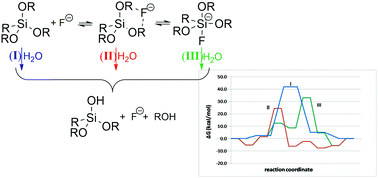
The hydrolysis reaction of methyltrimethoxysilane MeSi(OMe)3 under neutral conditions and the catalytic role of the fluoride ion in this reaction have been re-examined using DFT theoretical methods in methanol solution. Contrary to the common opinion, formation of pentacoordinate silicon fluoride, either free or complexed with a water molecule, proceeds with a free energy barrier. A hypercoordinate silicon intermediate is in equilibrium with the ion–dipole complex with fluoride, which is usually lower in energy than the pentacoordinate species. A comparison of the hydrolysis pathways of uncomplexed silanes, MeSi(OMe)3−n(OH)n, n = 0–3, and the corresponding ion–dipole complexes with fluoride and pentacoordinate silicon fluorides showed that both complexes hydrolyze more easily than the uncomplexed silanes. The pathway involving ion–dipole complexes is kinetically preferred. The catalytic effect of fluorine in the hydrolysis of these complexes is of a basic nature, i.e., fluoride acts as a base, withdrawing a proton from the attacking water, thus increasing its nucleophilicity. However, since both intermediates are in equilibrium, nucleophilic catalysis may become significant under specific conditions, when the concentration of the pentacoordinate silicon–fluoride complex is higher.


 Please wait while we load your content...
Please wait while we load your content...