Development of novel triazole based dendrimer supported spiroborate chiral catalysts for the reduction of (E)-O-benzyl oxime: an enantioselective synthesis of (S)-dapoxetine†
Abstract
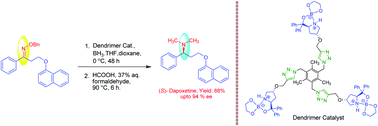
Novel dendrimer supported spiroborate catalysts 2 and 3 have been synthesized using a click reaction as a key step. The catalytic efficiency of the catalysts have been verified with reduction of (E)-O-benzyl oxime 13 as a model substrate. Catalyst 3 was found to be better than catalyst 2 as the chemical yield and enantiomeric excess were significantly high with the former catalyst. Thus, catalyst 3 has been successfully used in the efficient synthesis of (S)-dapoxetine 14 with 94% ee and 46% overall yield in three steps. These catalysts could be easily recovered from the reaction solution by the solvent precipitation technique and could be reused five times without significant loss of activity and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...