Stabilizing cathodes of lithium–sulfur batteries by the chemical binding of sulfur and their discharge products to carbon nanofibers†
Abstract
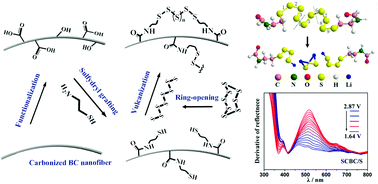
The reduction of elemental sulfur to lithium sulfides is a complicated process and it involves a series of redox reactions; this leads to a variety of dominating obstructions for the commercial applications of lithium–sulfur (Li–S) batteries. Herein, we improved the performance of lithium–sulfur batteries by covalently immobilizing the discharge products on carbon nanofibers by using cysteamine as a medium, which enabled us to achieve unprecedented capacity retention of ∼87.6% after 500 cycles at a current rate of 1C (1C = 1675 mA g−1) with high sulfur loading of 4.5 mg cm−2. Significantly, in situ UV/vis spectroscopy and X-ray absorption spectroscopy (XAS) of the electrolyte offered direct evidence for the proposed mechanism. No detectable signals of dissoluble polysulfides in the electrolyte were observed at any intermediate states, indicating the effect of covalent bonds on the reduction of long-chain polysulfide Li2Sx (4 < x ≤ 8) production. Theoretically, DFT calculations indicated the presence of different S–S bond lengths in linear polysulfanes and verified the presence of short-chain polysulfide Li2Sx (1 ≤ x ≤ 4) intermediates during discharge. This strategy provides an important way to solve one of the essential issues limiting the commercial applications of Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...