Self-assembly with fluorescence readout in a free base dipyrrin–polymer triggered by metal ion binding in aqueous solution†
Abstract
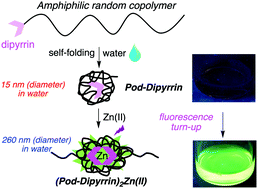
A hydrophobic dipyrrin has been attached to a heterotelechelic amphiphilic random copolymer as part of a single-polymer–single-cargo strategy. The polymer–dipyrrin (“Pod-dipyrrin”) exhibits an extended conformation in the organic solvent N,N-dimethylformamide (DMF) but in aqueous solution forms a unimeric nanoparticle (i.e., a foldamer) as indicated by dynamic light-scattering (DLS) data (hydrodynamic diameter Dh = 140 nm versus 15 nm in the two solvents, respectively). Treatment of the Pod-dipyrrin with Zn(II) in aqueous solution causes formation of a bis(Pod-dipyrrinato)Zn(II) complex as evidenced by a (1) slight change in absorption spectrum, (2) ∼50-fold increase in fluorescence quantum yield (Φf from 0.0035 to 0.17), and (3) profound increase in Dh (from 15 nm to 260 nm). The Dh value for the bis(Pod-dipyrrinato)Zn(II) complex is only slightly smaller in water (260 nm) than in DMF (310 nm), where a more extended conformation is expected. The increase in fluorescence of the bis(Pod-dipyrrinato)Zn(II) complex is readily observed by visual inspection (i.e., naked-eye readout). The conversion of a compact folded unimer (15 nm) to a more extended dimer (260 nm) triggered by metal ion addition denotes a conformationally malleable, environmentally sensitive molecular architecture. The self-assembly process can be reversed upon treatment with a metal-ion sequestering agent such as EDTA. The facile synthesis of a fluorogenic architecture that undergoes profound spontaneous change in molecular morphology upon binding an exogenous agent in water under physiological conditions may open a number of opportunities for sensory-mechanical studies in the life sciences.


 Please wait while we load your content...
Please wait while we load your content...