Two alkali calcium borates exhibiting second harmonic generation and deep-UV cutoff edges†
Abstract
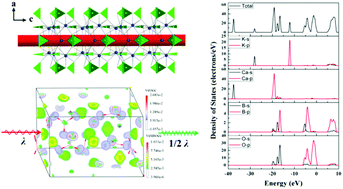
Two noncentrosymmetric alkali calcium borates KCa4(BO3)3 and K0.59Rb0.41Ca4(BO3)3 were synthesized by the high temperature solution method. They both crystallize in the polar space group Ama2 (No. 40). According to our investigation, KCa4(BO3)3 is the only potassium calcium borate and K0.59Rb0.41Ca4(BO3)3 is the first potassium rubidium calcium borate. Two borates both exhibit deep-UV cutoff edges (<200 nm) and moderate second harmonic generation (SHG) responses. In this paper, the detailed summaries of B–O connection styles in the disorder-free alkali- and alkaline-earth-metal borates have been discussed. Relationships between metal cations and boron atoms are studied. In addition, the optical and thermal property characterization of the two borates was systematically studied. To better understand the structure–property relationships, ab initio density functional theory was employed for the calculation of the optical refractive indices, birefringence and SHG coefficients. Additionally, the SHG density method was used to further explore the spatial distribution of the electronic states dominating the NLO activity.


 Please wait while we load your content...
Please wait while we load your content...