Sulfated zirconia: an efficient catalyst for the Friedel–Crafts monoalkylation of resorcinol with methyl tertiary butyl ether to 4-tertiary butylresorcinol†
Abstract
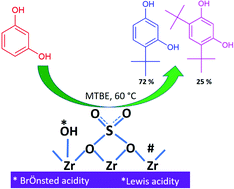
Friedel–Crafts alkylation of resorcinol with methyl tertiary butyl ether was carried out over sulfated zirconia (SZ) catalysts in the liquid phase. The SZ catalysts were synthesized by an impregnation method with different sulfur amounts and characterized by XRD, FT-IR, nitrogen sorption, XPS, SEM, pyridine-FTIR, and NH3-TPD. The effect of the sulfur loading on the total acidity and catalytic activity was investigated. The influence of the nature of the solvents on the alkylation reaction was inspected in terms of their acceptor and donor numbers. The sulfur loading, amount of solvent, temperature, catalyst amount, mole ratio and reusability of the catalyst were examined. The SZ catalyst synthesized by impregnating 1 N sulfuric acid was found to be highly selective for the monoalkylation to 4-tertiary butyl resorcinol (72%) with a resorcinol conversion of ∼70%. The catalyst was recycled thrice with a negligible decrease in the yield for 4-tertiary butylresorcinol. The SZ exhibited the best performance at low temperature (60 °C) among the different types of solid acid catalysts studied so far.


 Please wait while we load your content...
Please wait while we load your content...