A hydrazono-quinoline-based chemosensor sensing In3+ and Zn2+via fluorescence turn-on and ClO−via color change in aqueous solution†
Abstract
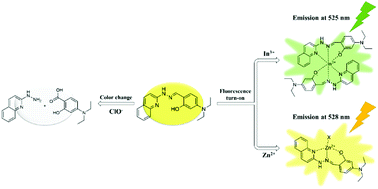
A new multi-target sensor, HQD ((E)-5-(diethylamino)-2-((2-(quinolin-2-yl)hydrazono)methyl)phenol), based on the hydrazono-quinoline moiety, was developed. Sensor HQD showed selectivity toward In3+ and Zn2+via fluorescence turn-on and ClO−via color change from yellow to colorless. The respective limits of detection for In3+, Zn2+ and ClO− were calculated to be 0.05, 0.08 and 3.10 μM, and were far below the WHO standard for zinc in drinking water (76.5 μM). Moreover, quantification of In3+ and Zn2+ was possible in real water samples. The processes of detection of In3+, Zn2+ and ClO− by HQD were demonstrated with the analyses of ESI-MS data, Job's plots, UV-Vis and 1H NMR titrations and computational studies.


 Please wait while we load your content...
Please wait while we load your content...