Folate-targeting and bovine serum albumin-gated mesoporous silica nanoparticles as a redox-responsive carrier for epirubicin release†
Abstract
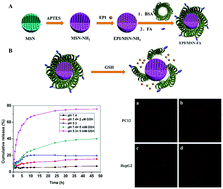
Herein, distinctive nanomaterial-based mesoporous silica nanoparticles (MSNs) successively coupled with bovine serum albumin (BSA) and folic acid (FA) were engineered for targeting the release of epirubicin (EPI). The BSA molecule as a “gatekeeper” prevents untimely drug leakage until the BSA coating layer is biodegraded in response to GSH. FA promotes the specific intracellular delivery of the drug to high folate receptor (FR)-expressing cancer cells to improve the efficacy of chemotherapy. In vitro drug release profiles show that this drug carrier can avoid premature release of the drug under physiological conditions, and redox-responsive EPI release from EPI/MSN-FA with easy biodegradability is significantly accelerated in the presence of GSH due to the cleavage of the intramolecular disulfide bond; thus, a “redox-triggered release” of the drug in the simulated targeted tissue microenvironment is achieved. The folate-mediated cellular uptake measured by confocal laser scanning microscopy (CLSM) shows that the hybrid nanoparticles more likely enter the FR-positive (FR+) HepG2 cells than the FR-negative (FR−) PC-12 cells. Toxicity experiment also demonstrates the negligible toxicity of MSN-FA and an increase in the antitumor suppression of EPI/MSN-FA with an increase in the dosage. The results show that the strategy of combining mesoporous silica with protein molecules having stimuli-responsive biodegradability will offer new potential directions towards the “on-demand” drug release for pinpoint chemotherapy in cancer treatment.


 Please wait while we load your content...
Please wait while we load your content...