Efficient synthesis of novel RGD based peptides and the conjugation of the pyrazine moiety to their N-terminus†
Abstract
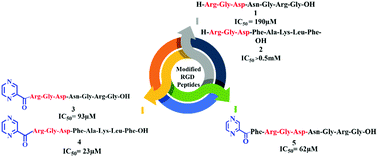
The synthesis of modified RGD peptides and their conjugation to the pyrazine skeleton at their N-terminus are described. To modify and alter the RGD sequence, short bioactive peptides such as FALKF and NGRG were added to the RGD N-terminus. Moreover, the in vitro investigation of these modified peptides, by a cell adhesion assay using the melanoma cell lines M21 expressing αvβ3 and M21L lacking αv expression, was made and interestingly peptide 4 containing the pyrazine moiety with the linear RGDFAKLF sequence gave the best IC50 value with M21 and all the peptides were unable to bind to M21L, demonstrating the selective recognition to αvβ3. The results showed pyrazine plays an essential role in the activity of the peptides. From these results, we can suggest that these peptides can affect cancer cells by abrogation of cell adhesion (cell migration).


 Please wait while we load your content...
Please wait while we load your content...