Functionalization of gold nanoparticles with two aminoalcohol-based quinoxaline derivatives for targeting phosphoinositide 3-kinases (PI3Kα)†
Abstract
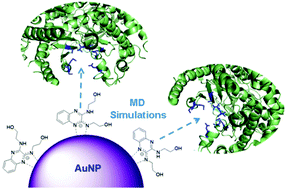
Quinoxaline derivatives have attracted considerable attention due to their vast range of applications that includes electroluminescence and biomedicine. Concerning the latter, the literature has shown that compounds with a quinoxaline motif bind quite efficiently to phosphatidylinositol-4,5-bisphosphate 3-kinases (PI3Ks), which are enzymes found to be overexpressed in some types of neoplasms. In the present study, gold nanoparticles (AuNPs) were easily functionalized with 2,3-diethanolminoquinoxaline (DEQX) and 2-(2,3-dihydro-[1,4]oxazino[2,3-b]quinoxalin-4-yl)ethanol (OAQX). We made use of glycerol in alkaline media as reducing agent and the quinoxalines served as capping ligands to stabilize the AuNPs. This is the first report on the modification of a nanostructure with quinoxalines. Functionalization confers nanoparticles the required specificity to target only cancer cells, which opens possibilities for phototherapy since the modified AuNPs would concentrate in the tumor tissue as a consequence of PI3Kα overexpression. Molecular dynamics simulations have shown that DEQX and OAQX are potential inhibitors of PI3Kα since they bind to the active site of the enzyme in a way similar to other known inhibitors.


 Please wait while we load your content...
Please wait while we load your content...