Metal-free, visible-light-promoted oxidative radical cyclization of N-biarylglycine esters: one-pot construction of phenanthridine-6-carboxylates in water†
Abstract
A metal-free, visible-light (blue LED: hν = 425 ± 15 nm) photoredox-catalyzed intramolecular cyclization reaction of N-biarylglycine esters to phenanthridine-6-carboxylates in water under an open air atmosphere at ambient conditions has been developed. A plausible mechanism is proposed for the reaction. Using a catalytic amount (5 mol%) of rose bengal and a blue LED, the N-biarylglycine esters were transformed into radical intermediates, which then underwent an intramolecular cyclization reaction followed by dehydrogenation in one-pot to give the desired products in up to 93% yield. This approach is pertinent for the gram-scale synthesis, eco-friendliness and atom economy as compared to reported methods for the synthesis of phenanthridines. Moreover, to the best of our knowledge, no instance has hitherto been described on the visible-light photocatalyzed transformation of inexpensive and biocompatible N-biarylglycine esters to phenanthridines.
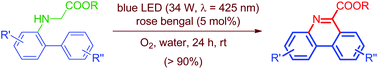


 Please wait while we load your content...
Please wait while we load your content...