Efficient synthesis of organic semiconductors by Suzuki–Miyaura coupling in an aromatic micellar medium†
Abstract
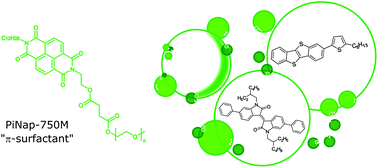
Micellar catalysis enables carrying out Suzuki–Miyaura couplings in water under exceptionally mild conditions. Extension of such a protocol to the sustainable synthesis of highly conjugated, poorly soluble materials like [1]benzothieno[3,2-b][1]benzothiophene (BTBT) requires redesigning of the surfactants employed. The here reported new naphthalenediimide containing amphiphilic derivative, PiNap-750M, features unprecedented performances in the preparation of this and other relevant classes of organic semiconductors in water and at room temperature.


 Please wait while we load your content...
Please wait while we load your content...