Direct electrosynthesis for N-alkyl-C3-halo-indoles using alkyl halide as both alkylating and halogenating building blocks†
Abstract
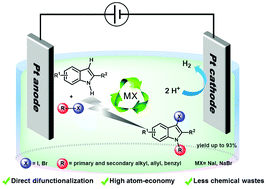
An electrochemically induced tandem reaction has been developed for selective N1-alkylation and C3-halogenation of indoles. This electrochemical difunctionalization strategy circumvents conventional multi-step procedures and efficiently generates synthetically important N-alkyl-3-halo-indoles under more environmentally benign conditions. This reaction can proceed in a simple undivided cell, without the use of any oxidant, base or transition-metal. The excellent atom-economy of this method is highlighted by fully using alkyl halide as both alkylating and halogenating building blocks without atom waste.


 Please wait while we load your content...
Please wait while we load your content...