Selectivity control of Pd(PMe3)4-catalyzed hydrogenation of internal alkynes to E-alkenes by reaction time and water content in formic acid†
Abstract
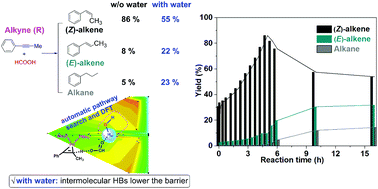
The modulation of selectivity of transfer hydrogenation of alkynes to E-alkenes using formic acid is a challenge due to the limited knowledge of the complex reaction network, including oxidative addition, decarboxylation, reductive elimination, Z → E isomerization, and β-H elimination. Here, the search for the reaction pathway and experiment explorations revealed that the selectivity of Pd(PMe3)4-catalyzed hydrogenation of 1-phenyl-1-propyne to (E)-1-phenylpropene is controlled by the water content in the aqueous solution of formic acid and the reaction time. The combination of an automatic reaction pathway search and density functional theory (DFT) calculations found that the intermolecular hydrogen bonds with water molecules have an influence on lowering the free energy activation barrier of transition states in the oxidative addition steps. The reasonable reaction barriers of Z → E isomerization and hydrogenation result in the dependence of selectivity on reaction time, which has been supported by experiments. By using molecular sieves, the water in formic acid is removed, and the yield of the desired (Z)-1-phenylpropene product increases to the highest value (86%) in 5 hours but decreases to 54% when the reaction is run for 16 hours due to the further Z → E isomerization and hydrogenation. In the second stage which starts from (Z)-1-phenylpropene, the yield of (E)-1-phenylpropene decreased from 90% (with 4 Å MS) to 67% in the aqueous solution of formic acid.


 Please wait while we load your content...
Please wait while we load your content...