Spectroscopic speciation of aqueous Am(iii)–oxalate complexes†
Abstract
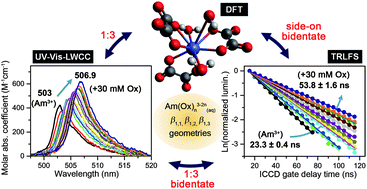
Transportation of actinides through the geosphere is facilitated by complexation with organic ligands dissolved in groundwater. Carboxylic groups can interact directly with actinide ions and are found among the most abundant organic ligands in alkaline aquatic systems like underground water. In this study, the complexation of organic carboxylic groups with Am(III) was investigated by monitoring the interactions of Am(III) with oxalate (Ox), the simplest dicarboxylate ligand, in solution. UV-Vis spectrophotometry coupled with a liquid waveguide capillary cell (100 cm optical path-length) and time-resolved laser fluorescence spectroscopy were employed for quantitative detection of the respective Am(III)–Ox species. Increasing the Ox concentration caused significant spectral changes, i.e., red-shifts in both the absorption and luminescence maxima with increased molar absorption coefficients, enhanced luminescence intensities, and prolonged luminescence lifetimes. Individual spectra of AmOx+(aq), Am(Ox)2−(aq), and Am(Ox)33−(aq) were resolved by deconvolution of the absorption spectra, with apparent formation constants of log β1,1 = 5.34 ± 0.05, log β1,2 = 9.14 ± 0.18, and log β1,3 = 11.49 ± 0.30, respectively, in I = 0.1 M NaClO4 and 0–30 mM Na2Ox. The absorption and luminescence spectral changes suggest bidentate complexation of Ox with Am(III) via inner-sphere interactions. The geometry of the Am(III)–Ox complexes was optimized by density functional theory, where the bonding characteristics were in good agreement with the experimental results. Thorough spectroscopic characterization enabled speciation of the Am(III)–Ox complexes and determination of their formation constants. This spectroscopic approach is generally applicable in the investigation of molecular interactions between Am(III) and various ligands in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...