Acid properties of organosiliceous hybrid materials based on pendant (fluoro)aryl-sulfonic groups through a spectroscopic study with probe molecules†
Abstract
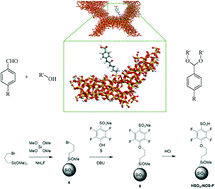
Two different heterogeneous catalysts carrying aryl-sulfonic moieties, in which the aromatic ring was either fluorinated or not, were successfully synthesized. The multi-step synthetic approaches implemented involved the synthesis of the silyl-derivative, template-free one-pot co-condensation (at low temperature and neutral pH) and tethering reaction. A multi-technique approach was implemented to characterize the hybrid organic–inorganic catalysts involving TGA, N2 physisorption analysis, FTIR spectroscopy, and ss MAS NMR (1H, 13C, 29Si) spectroscopy. Specifically, the acidity of the organosiliceous hybrid materials was studied through the adsorption of probe molecules (i.e. CO at 77 K and NH3 and TMPO at room temperature) and a combination of FTIR and ss MAS NMR spectroscopy. The catalytic activity of the two hybrids was tested in the acetal formation reaction between benzaldehyde and ethylene glycol. Preliminary results indicated superior performances for the fluoro-aryl-sulfonic acid, compared to the non-fluorinated sample. The findings hereby reported open new avenues for the design of heterogeneous sulfonic acids with superior reactivity in acid-catalyzed reactions. Moreover, through the implementation of spectroscopic studies, using probe molecules, it was possible to investigate in detail the acidic properties of hybrid organosiliceous materials.


 Please wait while we load your content...
Please wait while we load your content...