Computational study on the 1,3-diyne synthesis from gold(i)-catalyzed alkynylation of terminal alkynes with alkynyl hypervalent iodine reagents under the aid of a silver complex and 1,10-phenanthroline†
Abstract
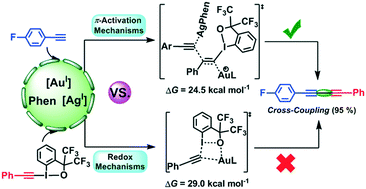
The gold(I)-catalyzed cross-coupling reaction of aryl terminal alkynes with alkynyl hypervalent iodine(III) reagents presents a new strategy for the synthesis of unsymmetrical 1,3-diynes in the presence of AgOTs and 1,10-phenanthroline (Phen). With the aid of DFT calculations, the present study systematically investigated the mechanism of the target transformation. The results show a new Au/Ag co-catalyzed π-activation mechanism for formation of the unsymmetrical 1,3-diyne, which is remarkably different from the previously proposed redox mechanism by experimental authors. The new mechanism emphasizes that a silver(I) complex with a Phen ligand performs the C(sp)–H activation of terminal alkyne to generate the active silver acetylide intermediate, which then nucleophilically attacks the Cβ atom of the gold(I)-coordinated alkynyl hypervalent iodine reagent in the anti-mode to give the gold vinyl intermediate, and subsequently the unsymmetrical cross-coupling product is formed via a concerted α-elimination and 1,2-phenyl migration step. The calculated barrier is 24.5 kcal mol−1 for formation of the unsymmetrical 1,3-diyne. In comparison, the formation of the symmetrical 1,3-diyne follows the redox mechanism with a comparatively high barrier of 29.0 kcal mol−1. It is confirmed that the alkynyl hypervalent iodine(III) reagent plays dual roles: as an alkyne surrogate and also a chemical oxidant. In addition, the theoretical results reveal the vital roles of AgOTs and Phen, and rationalize the experimental observations of the decisive product originating from unsymmetrical cross-coupling reaction.


 Please wait while we load your content...
Please wait while we load your content...