Formic acid as a hydrogen source for the iridium-catalyzed reductive amination of levulinic acid and 2-formylbenzoic acid†
Abstract
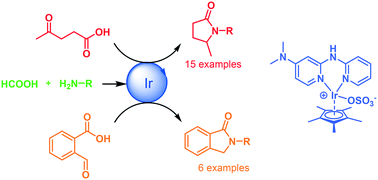
The aqueous phase catalytic reductive amination of levulinic acid (LA) into pyrrolidones and 2-formylbenzoic acid into N-arylisoindolines is reported. The catalytic reaction involves an iridium catalyst bearing an electron-rich dipyridylamine ligand and uses formic acid as a hydrogen source. The chemoselective iridium catalyst displayed high efficiency for the synthesis of a variety of N-substituted 5-methyl-2-pyrrolidones, N-substituted 6-methyl-2-piperidinones and N-arylisoindolinones.


 Please wait while we load your content...
Please wait while we load your content...