A new insight into SO2 low-temperature catalytic oxidation in porous carbon materials: non-dissociated O2 molecule as oxidant†
Abstract
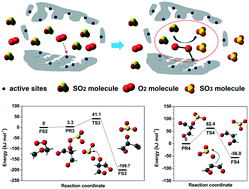
SO2 oxidation is the rate-determining step during the process of low-temperature SO2 conversion to high-value H2SO4 in porous carbon materials. Non-dissociated O2 molecules and chemisorbed O atoms from O2 dissociation are possible oxidants for SO2 oxidation. While the pathways of oxidation reactions in which chemisorbed O atoms participate have been previously studied via density functional theory calculation, the possible SO2 oxidation pathways by non-dissociated O2 remain to be investigated. Inspired by the mechanism in which a non-dissociated O2 molecule oxidizes other molecules such as CO and NO, we elucidate the detailed pathways for SO2 oxidation by non-dissociated O2 using density functional theory calculation assisted by SO2 removal experiment validation. Computational results show that non-dissociated O2 can be activated at the active sites of porous carbon, generating a C–O–O structure. By low-barrier O transfer processes, the formed C–O–O structure can directly oxidize gaseous SO2 to SO3 without the need of the SO2 chemisorption process. Transient response experiments further validate that the oxidant for SO2 conversion to SO3 in porous carbon materials is non-dissociated O2. This work reveals the role of non-dissociated O2 in directly oxidizing non-chemisorbed SO2 to SO3 at low temperature, providing a new understanding for SO2 conversion to SO3 in porous carbon materials.


 Please wait while we load your content...
Please wait while we load your content...