Cobalt-catalysed unactivated C(sp3)–H amination: two-state reactivity and multi-reference electronic character†
Abstract
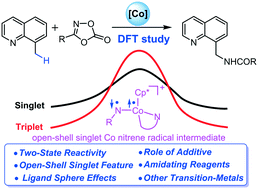
Theoretical calculations have been performed to gain deeper understanding on the mechanism of Cp*Co(III)-catalysed C(sp3)–H amination of 8-methylquinoline with phenyl-1,4,2-dioxazol-5-one. The result suggests that the additive AgSbF6 could thermodynamically facilitate the generation of catalytically active species Cp*Co(OPiv)+. The subsequent catalytic cycle involves sequential external base assisted C(sp3)–H activation, decarboxylation, nitrene insertion, and protonation. Importantly, a remarkable “two-state reactivity” scenario was disclosed for this reaction. A distinct multi-reference feature was found in the processes for Co-nitrene radical intermediate and C–N bond formations, which thus greatly enriches the cobalt catalysis chemistry. Such a multi-reference character was the outcome of double excitation from the Co–N π-bonding orbital to its antibonding orbital. DFT results indicate that aminating reagents with a rigid structure would be preferred because they form strong orbital interaction with the metal center. In addition, it is theoretically predicted that the Fe(III) species is a promising candidate for efficient C(sp3)–H activation and Tp (Tp = hydridotris(pyrazolyl)borate) can serve as a more effective ligand for Co(III) catalysed C(sp3)–H activation, thanks to its stronger interaction with the Co center which thus stabilizes the transition state.


 Please wait while we load your content...
Please wait while we load your content...