New insights into the effect of nitrogen incorporation in Mo: catalytic hydrogenation vs. hydrogenolysis
Abstract
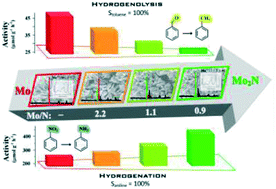
The catalytic effect of nitrogen incorporation into Mo on hydrogenation (of –NO2 to –NH2 in nitrobenzene to aniline) and hydrogenolysis (of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in benzaldehyde to toluene) processes has been assessed. Bulk Mo was prepared by temperature programmed reduction of MoO3 (in H2 to 933 K) and β-Mo2N (confirmed by powder XRD) subsequently synthesised by Mo nitridation in N2/H2. Two intermediate samples (MoN-1 and MoN-2) with different Mo/N ratio were prepared by altering the duration (1 and 2 h) of the nitridation step. XPS analysis revealed a nitrogen surface enrichment (Mo/N = 2.2 → 0.9 from MoN-1 to β-Mo2N) relative to the bulk (Mo/N = 5.1 → 2.5). Incorporation of N did not affect morphology and each sample exhibited (by SEM analysis) aggregates (<5 μm) of crystals (27–36 nm) with unchanged specific surface area (ca. 4 m2 g−1). Hydrogen chemisorption and release (by TPD) increased with decreasing Mo/N (Mo < MoN-1 < MoN-2 < β-Mo2N). Gas phase hydrogenation of nitrobenzene to aniline exhibited increasing rate from Mo → β-Mo2N, attributed to higher availability of surface heterolytic hydrogen (on Mo–N). In contrast, conversion of benzaldehyde to toluene was favoured by increasing Mo/N (from β-Mo2N → Mo) where hydrogenolytic –C
O in benzaldehyde to toluene) processes has been assessed. Bulk Mo was prepared by temperature programmed reduction of MoO3 (in H2 to 933 K) and β-Mo2N (confirmed by powder XRD) subsequently synthesised by Mo nitridation in N2/H2. Two intermediate samples (MoN-1 and MoN-2) with different Mo/N ratio were prepared by altering the duration (1 and 2 h) of the nitridation step. XPS analysis revealed a nitrogen surface enrichment (Mo/N = 2.2 → 0.9 from MoN-1 to β-Mo2N) relative to the bulk (Mo/N = 5.1 → 2.5). Incorporation of N did not affect morphology and each sample exhibited (by SEM analysis) aggregates (<5 μm) of crystals (27–36 nm) with unchanged specific surface area (ca. 4 m2 g−1). Hydrogen chemisorption and release (by TPD) increased with decreasing Mo/N (Mo < MoN-1 < MoN-2 < β-Mo2N). Gas phase hydrogenation of nitrobenzene to aniline exhibited increasing rate from Mo → β-Mo2N, attributed to higher availability of surface heterolytic hydrogen (on Mo–N). In contrast, conversion of benzaldehyde to toluene was favoured by increasing Mo/N (from β-Mo2N → Mo) where hydrogenolytic –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O scission is favoured by homolytic hydrogen chemisorption (on Mo). Our results provide the first evidence that N incorporation in Mo structure can control catalytic hydrogenation vs. hydrogenolysis performance.
O scission is favoured by homolytic hydrogen chemisorption (on Mo). Our results provide the first evidence that N incorporation in Mo structure can control catalytic hydrogenation vs. hydrogenolysis performance.


 Please wait while we load your content...
Please wait while we load your content...