Site energy distribution of sodium ions in a sodium rubidium borate glass
Abstract
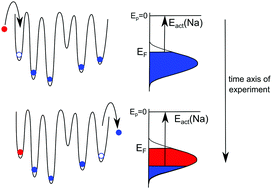
A charge attachment induced transport experiment has been conducted on a Na+ and Rb+ containing glass employing an external Rb+ ion beam. Native Na+ ions are replaced by external Rb+ ions giving rise to a pronounced concentration depth profile as measured by time-of-flight secondary ion mass spectrometry. From the theoretical analysis of this concentration profile a unique site energy distribution (SED) of mobile Na+ ions in the glass is derived. The full width at half maximum of the populated part of this SED is 0.32 eV. The mechanism involves Na+ sites being vacated top-down and being filled by Rb+ also top down. Therefore, the Fermi energy of Na+ ions decreases with ongoing experiment, while that of the Rb+ ions stays constant. Agreement between experiment and the Nernst–Planck–Poisson theory for describing the transport is reached by assuming that both the migration and the chemical diffusion driven contribution to the total flux depend on the local concentration.


 Please wait while we load your content...
Please wait while we load your content...