How carotenoid distortions may determine optical properties: lessons from the Orange Carotenoid Protein†
Abstract
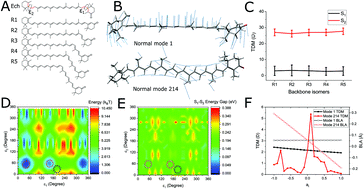
Carotenoids in photosynthetic proteins carry out the dual function of harvesting light and defending against photo-damage by quenching excess energy. The latter involves the low-lying, dark, excited state labelled S1. Here “dark” means optically-forbidden, a property that is often attributed to molecular symmetry, which leads to speculation that its optical properties may be strongly-perturbed by structural distortions. This has been both explicitly and implicitly proposed as an important feature of photo-protective energy quenching. Here we present a theoretical analysis of the relationship between structural distortions and S1 optical properties. We outline how S1 is dark not because of overall geometric symmetry but because of a topological symmetry related to bond length alternation in the conjugated backbone. Taking the carotenoid echinenone as an example and using a combination of molecular dynamics, quantum chemistry, and the theory of spectral lineshapes, we show that distortions that break this symmetry are extremely stiff. They are therefore absent in solution and only marginally present in even a very highly-distorted protein binding pocket such as in the Orange Carotenoid Protein (OCP). S1 remains resolutely optically-forbidden despite any breaking of bulk molecular symmetry by the protein environment. However, rotations of partially conjugated end-rings can result in fine tuning of the S1 transition density which may exert some influence on interactions with neighbouring chromophores.


 Please wait while we load your content...
Please wait while we load your content...