Static and dynamic scavenging of ammoniated electrons by nitromethane†
Abstract
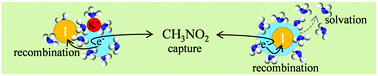
We studied the time-resolved scavenging efficiency of nitromethane for transient electron species in liquid ammonia, at a temperature of 298 K. UV excitation of iodide ions produced fully solvated electrons, as well as transient (I, e−) and (counterion, e−) pairs, the overall concentration of which was monitored by NIR absorption with subpicosecond time resolution. After the UV pulse, the solution absorbance decays almost completely in a few hundreds of picoseconds due to geminate electron–iodine atom recombination and a competitive annihilation channel involving the scavenger. Recombination of transient (I, e−) pairs follows the well-known kinetic model, while the electron–nitromethane reaction proceeds by two distinct mechanisms: static scavenging (interpreted in terms of the encounter complex model), with a characteristic time shorter than the temporal resolution of the apparatus, or via a diffusion-limited bimolecular reaction, with a rate constant of 1.1 × 1011 M−1 s−1.


 Please wait while we load your content...
Please wait while we load your content...