Deciphering the grounds of the suitability of acylhydrazones as efficient photoswitches†
Abstract
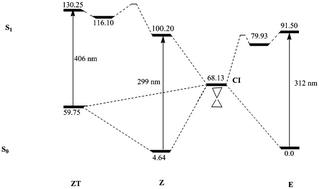
Many efforts are currently being devoted to designing molecular photoswitches with specific properties. In this sense, a recent publication [D. J. van Dijken et al., J. Am. Chem. Soc., 2015, 137, 14982–14991] has synthesized and analyzed the photochromic properties of a large set of acylhydrazones (ACHs), a relatively unexploited class of potential photoswitches with two stable E and Z isomers. This study has revealed a very diverse and complex pattern of the absorption/emission properties of ACHs depending on the substituents attached to the ACH motif. In this work, high level theoretical calculations are performed on a representative set of the experimentally studied ACHs in order to analyze, at the molecular level, the reasons behind the different photochemistries experimentally observed. This systematic study allows for the classification of the full set of ACHs into just four categories. The two more common groups display a small, either positive or negative, shift of the maximum wavelength of absorption between the E and Z isomers. Less common, but far more interesting from a practical point of view, are the compounds that show a large (>100 nm) Stokes shift. This behavior may arise from two different situations. The most common one implies the possibility of an intramolecular proton transfer in the excited electronic state of the less stable Z isomer. The less likely scenario would also involve a loss of the azidic proton through an intermolecular proton transfer that would take place with the aid of the solvent.


 Please wait while we load your content...
Please wait while we load your content...