Structural and dynamical heterogeneities at glutamine–water interfaces†
Abstract
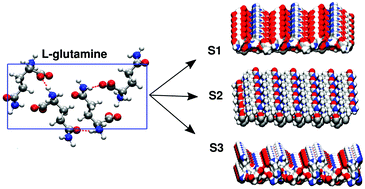
The behavior of water at the surfaces of solid amino acid crystals has received little attention despite its importance in nucleation processes. In this work, we take a first step to fill this gap by using molecular dynamics simulations to study the structural and dynamical properties of water near the (100), (010) and (001) surfaces of L-glutamine crystals. These highly hydrophilic surfaces serve as excellent model systems for interrogating the behavior of water. Despite having the same molecular composition, water at each surface displays characteristic structural, orientational and dynamical correlations. This behavior is tuned by how the different chemical groups of amino acids make contact with the liquid phase. All three surfaces yield a glassy layer of interfacial water which is reflected in different ways such as the presence of a rotationally arrested layer of water molecules and substantial slow down of the diffusion of water near the interface. By increasing the concentration of molecules in solution, we show that the binding of glutamine molecules to the crystal surface creates a crowded environment involving pockets of trapped water molecules altering the water dynamics in a highly non-trivial manner suggesting that the solvent dynamics may have important implications on crystal nucleation.


 Please wait while we load your content...
Please wait while we load your content...