Notable effect of water on excess electron attachment to aqueous DNA deoxyribonucleosides†
Abstract
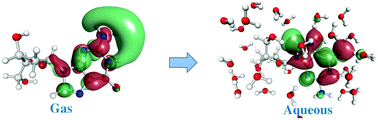
Computations using the combined quantum mechanical/molecular mechanical (QM/MM) method were performed to investigate excess electron attachment to and detachment from aqueous deoxyribonucleosides (dRNs). The QM/MM vertical electron affinities (VEAs) of four dRNs are higher than the values of the corresponding nucleobases by ∼0.20 eV. The QM/MM diabatic electron affinities (AEAs) are much larger than the calculations of the implicit solvent model. Bulk water induces evident VEA and AEA increases and boosts the vertical detachment energies by over 1.20 eV. It affects excess electron attachment to and detachment from aqueous dRNs and stabilizes the anions. Moreover, the water molecules around deoxyadenosine (dA) anions form intermolecular hydrogen bonds with dA and break the intramolecular hydrogen bond of dA which had been found in the gas structure. In vertical electron attachment, ∼50% of excess electrons would be delocalized over the water molecules around the dRNs. The anionic structural relaxations cause the transfer of ∼−0.30 e excess electrons from the water molecules to the dRN nucleobases. However, the main excess electrons (∼−0.76 e) would be localized on dRN nucleobases in the stable anionic structure.


 Please wait while we load your content...
Please wait while we load your content...