In situ cryocrystallization and solid-state structures of furfural and some derivatives†
Abstract
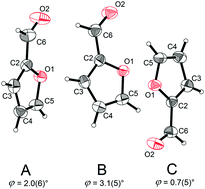
The crystal and molecular structures of the bio-based platform chemical furfural (1) and its derivatives furfurylamine (2), 2-furonitrile (3) and 2-methylfuran (4), which are all liquid at ambient temperature, have been determined by in situ cryocrystallography. Compound 1 crystallizes with three molecules in the asymmetric unit (Z′ = 3), which all adopt the syn conformation. Compound 2 crystallizes with two crystallographically distinct molecules in gauche and anti conformers, which are linked by N–H⋯N and N–H⋯O hydrogen bonds. The crystal structure of 3 is characterized by C–H⋯N interactions between C4 of the furan ring and the nitrile group. Compound 4 was found to crystallize in the non-centrosymmetric space group P42bc with a polar c axis. The molecular structures are compared with results from spectroscopic studies in the literature. Compounds 1–4 show a difference of ca. 14° between the external C–C–C and C–C–O angles to the substituent carbon atom in 2-position of the furan ring. Using this criterion as an indicator for the assignment of the oxygen atom in 2-substituted furan derivatives, a number of crystal structures in the Cambridge Structural Database (CSD) are called into question.


 Please wait while we load your content...
Please wait while we load your content...