Formation of maghemite nanostructures in polyol: tuning the particle size via the precursor stoichiometry†
Abstract
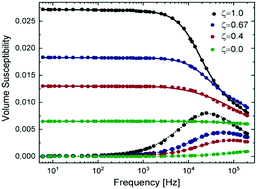
This study investigates a thermal synthesis in which iron(II) and iron(III) chlorides are used to form maghemite nanoflowers in the presence of sodium hydroxide and a solvent mixture of N-methyldiethanolamine and diethylene glycol. The agglomeration process leading to nanoflower formation is examined by testing temperatures of synthesis ranging from 180 °C to 220 °C and holding times from 2 h to 12 h. A temperature of 220 °C and a holding time of 2 h are confirmed to be suitable synthesis parameters for nanoflower formation. The process of primary particle ordering during agglomeration leading to cooperative magnetic behaviour is discussed. The stoichiometric ratio of Fe2+ and Fe3+ ions was varied in the precursor solution and it is shown to have a strong influence on particle and crystallite sizes, and thereby on the magnetic properties. A larger Fe3+ ion content during the synthesis leads to larger particles and crystallites, while a higher content in Fe2+ ions favours nucleation at the expense of growth. An additional treatment with iron nitrate leads to further growth of crystallites as well as particles. Stable, mostly monodisperse suspensions of multicore particles with diameters ranging from 18.4 nm to 28.7 nm show Brownian relaxation times between 0.2 μs and 9 μs and dynamic susceptibilities at 25 kHz from about 7 × 10−3 m3 kg−1 [Fe] to 20 × 10−3 m3 kg−1 [Fe], making the particles interesting candidates for magnetic hyperthermia and magnetic particle imaging.


 Please wait while we load your content...
Please wait while we load your content...