Development of 4-oxime-1,8-naphthalimide as a bioorthogonal turn-on probe for fluorogenic protein labeling†
Abstract
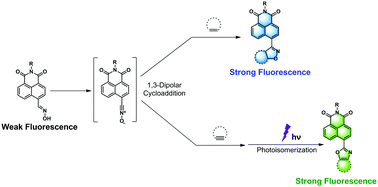
4-Oxime-1,8-naphthalimide was reported as a novel bioorthogonal turn-on probe based on 1,3-dipolar cycloaddition reactions between in situ generated nitrile oxide and alkenes/alkynes. The resulting isoxazole products displayed dramatically strong fluorescence enhancement upon photoirradiation through isoxazole–oxazole photoisomerization. This new methodology was successfully applied for in situ fluorogenic protein labeling.


 Please wait while we load your content...
Please wait while we load your content...