N-Heterocyclic carbene-catalyzed β-addition of enals to 3-alkylenyloxindoles: synthesis of oxindoles with all-carbon quaternary stereocenters†
Abstract
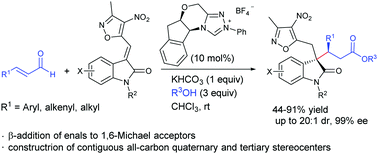
The N-heterocyclic carbene-catalyzed β-addition of enals to 3-alkylenyloxindoles was developed. All of the β-aryl, β-alkenyl and β-alkyl enals worked well for the reaction to give the corresponding 3,3-disubstituted oxindoles bearing contiguous all-carbon quaternary and tertiary stereocenters in good yields with moderate to high diastereoselectivities and excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...