Ring-opening cyclization of spirocyclopropanes with stabilized sulfonium ylides for the construction of a chromane skeleton†
Abstract
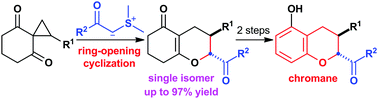
Regioselective ring-opening cyclization of cyclohexane-1,3-dione-2-spirocyclopropanes with stabilized sulfonium ylides provided 2,3-trans-disubstituted 2,3,4,6,7,8-hexahydro-5H-1-benzopyran-5-ones in high yields without the formation of any isomers. The obtained product was readily converted into highly substituted chromane.


 Please wait while we load your content...
Please wait while we load your content...