Direct oxidative dearomatization of indoles: access to structurally diverse 2,2-disubstituted indolin-3-ones†
Abstract
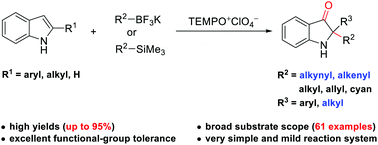
Described is an efficient oxidative dearomatization of indoles with TEMPO oxoammonium salt and a broad range of nucleophiles. Under very mild conditions, dearomative oxyalkynylation and oxyalkenylation of indoles to structurally diverse 2,2-disubstituted indolin-3-ones were developed for the first time with high atom-economy. In addition, dearomative oxyarylation, oxyallylation, and oxycyanation of indoles were also demonstrated. Mechanism studies indicated that TEMPO oxoammonium salt served as the sole oxidant source through an electron donor–acceptor (EDA) intermediate.


 Please wait while we load your content...
Please wait while we load your content...