Enantioenrichment of racemic BINOL by way of excited state proton transfer†
Abstract
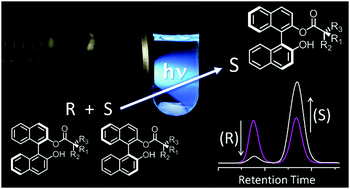
Here we report a method for enantioenriching BINOL using a chiral auxiliary and an excited state proton transfer (ESPT) event. Regardless of the starting enantiomeric excess (ee), after irradiation the solution reaches a photostationary state whose ee is dependent solely on the identity of the chiral auxiliary group. The enantioenriched BINOL is easily recovered by cleaving the auxiliary group in mild conditions.


 Please wait while we load your content...
Please wait while we load your content...