Simultaneous determination of cinobufagin and its five metabolites in rat plasma by LC-MS/MS for characterization of metabolic profiles and pharmacokinetic study†
Abstract
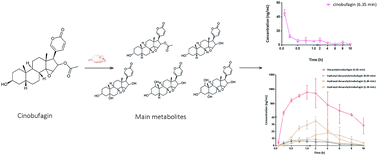
Cinobufagin is one of the main ingredients of Venenum Bufonis and has various pharmacological activities and toxicities. It is very important to simultaneously determine cinobufagin and its metabolites because of its strong physiological activity but it is also very challenging due to its low concentration in blood. In this study, a convenient and efficient method was developed to simultaneously determine cinobufagin and its five metabolites in rat plasma (semi-quantification). BF211, the derivative of bufalin, was added to the plasma sample as an internal standard (IS). An ACQUITY BEH C18 column (2.1 mm × 50 mm, 1.7 μm) was used for the separation at 40 °C, and the mobile phase containing the acetonitrile and water added 0.05% formic acid was run under gradient elution at 0.4 mL min−1. A LC-MS/MS with an electrospray ionization source in positive ion mode was used for the quantification of cinobufagin and its five metabolites in the mode of multiple reaction monitoring (MRM). The monitored ion pairs were m/z 443.5 → 365.3 for the transition of cinobufagin, m/z 513.7 → 145.3 for IS, and m/z 443.2 → 365.2, 401.2 → 265.2, and 417.2 → 363.2 for 3-epi-cinobufagin, desacetylcinobufagin and hydroxyl-desacetylcinobufagin respectively. The calibration curve of cinobufagin showed good linearity in the range of 1.0–200 ng mL−1 (y = 0.201x + 0.105 and R2 > 0.990) and the limit of quantitation (LOQ) was 1.0 ng mL−1. The specificity, accuracy, precision, extraction recovery, matrix effect and stability of the method were satisfactory. According to the results of pharmacokinetic study, cinobufagin was absorbed quickly (Tmax = 0.083 ± 0 h) and the main metabolite was desacetylcinobufagin (Cmax = 897.95 ± 237.35 ng mL−1). This pharmacokinetic study could be used for obtaining essential data to interpret the pharmacokinetic–pharmacodynamic relationships of cinobufagin.


 Please wait while we load your content...
Please wait while we load your content...