Bis-pyrene probes of foldamer conformation in solution and in phospholipid bilayers†
Abstract
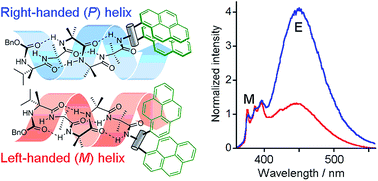
Exploring the detailed structural features of synthetic molecules in the membrane phase requires sensitive probes of conformation. Here we describe the design, synthesis and characterization of bis(pyrene) probes that report conformational changes in membrane-active dynamic foldamers. The probes were designed to distinguish between left-handed (M) and right-handed (P) screw-sense conformers of 310-helical α-aminoisobutyric acid (Aib) peptide foldamers, both in solution and in bilayer membranes. Several different bis(pyrene) probes were synthesized and ligated to the C-terminus of Aib tetramers that had different chiral residues at the N-terminus, residues that favored either an M or a P screw-sense in the 310-helix. The readily synthesized and conveniently incorporated N-acetyl-1,2-bis(pyren-1′-yl)ethylenediamine probe proved to have the best properties. In solution, changes in foldamer screw-sense induced substantial changes in the ratio of excimer/monomer fluorescence emission (E/M) for this reporter of conformation, with X-ray crystallography revealing that opposite screw-senses produce very different interpyrene distances in the reporter. In bilayers, this convenient and sensitive fluorescent reporter allowed, for the first time, an investigation of how the chirality of natural phospholipids affects foldamer conformation.


 Please wait while we load your content...
Please wait while we load your content...