Towards a continuous formic acid synthesis: a two-step carbon dioxide hydrogenation in flow†
Abstract
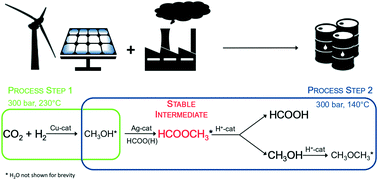
The need for long term, large-scale storage solutions to match surplus renewable energy with demand drives technological innovation towards a low-carbon economy. As a high hydrogen density energy carrier, formic acid streamlines functional storage of unscheduled intermittent power supply. However, the unfavourable thermodynamics of its direct synthesis from CO2 and H2 call for alternative processes to achieve substantial space time yields. This preliminary study investigates the feasibility of continuously producing formic acid in a two-step process by exploiting methyl formate as a thermodynamically stable intermediate. In order to prove the concept, the qualitative efficiency of several three-reactor configurations is evaluated and discussed with respect to the efficiency of a single reactor methanol synthesis over a commercial Cu catalyst. Although concrete solutions are not available yet and identification of formic acid remains arduous, the proposed reactive pathway exceeds the thermodynamic limits of the direct synthesis path over heterogeneous catalysts, and opens up avenues for advances in clean energy production.


 Please wait while we load your content...
Please wait while we load your content...