l-Proline catalyzed one-pot synthesis of polysubstituted pyridine system incorporating benzothiazole moiety via sustainable sonochemical approach†
Abstract
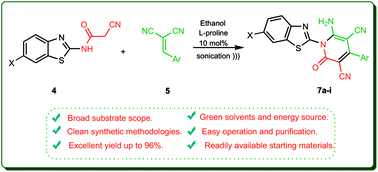
The efficient, highly convenient synthesis of polysubstituted pyridine derivatives was established via the reaction of N-(benzothiazol-2-yl)-2-cyanoacetamides with an assortment of arylidene malononitriles and arylidene ethyl cyanoacetates in the presence of L-proline as an efficient organocatalyst for this type of ultrasonic-mediated Michael addition. The mechanistic pathway and factors affecting this reaction were also established. The main characteristics of this procedure are high yields, use of a cost-effective catalyst, and easy work-up and purification.


 Please wait while we load your content...
Please wait while we load your content...