Influence of residual sodium ions on the structure and properties of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)†
Abstract
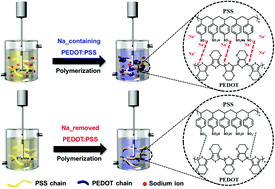
Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) is a promising conducting polymer in terms of its applicability to transparent and flexible electronic devices. Generally, a negatively charged PSS chain can interact with alkali metal cations like sodium and potassium. During polymerization, these ions, especially sodium ions, remain in an aqueous state and affect particle formation. This paper describes the effect of residual sodium ions on the synthesis of PEDOT:PSS and its electrical and optical properties. Removing the sodium ions weakens the coulombic interaction between the PEDOT and PSS chains, which leads to a linear conformation. This conformational change enhances the electrical conductivity and work function. Furthermore, transmittance in the visible region increased remarkably because the intrinsic electrical properties of the PEDOT:PSS particles were improved. Moreover, the colloidal stability was enhanced because the particle coagulation caused by residual sodium ions was reduced. In summary, we determined that sodium ions in PEDOT:PSS have a considerable influence on its electrical and optical properties and colloidal stability for practical applications.


 Please wait while we load your content...
Please wait while we load your content...