Tailoring the molecular design of twisted dihydrobenzodioxin phenanthroimidazole derivatives for non-doped blue organic light-emitting devices†
Abstract
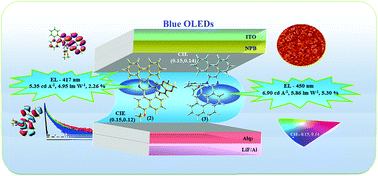
Three fused polycyclic aryl fragments, namely, naphthyl, methoxynaphthyl, and pyrenyl have been used to construct blue-emissive phenanthroimidazole-functionalized target molecules, i.e., 1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(naphthalen-1-yl)-1H-phenanthro[9,10-d]imidazole (1), 1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(1-methoxynaphthalen-4-yl)-1H-phenanthro[9,10-d]imidazole (2), and 1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(pyren-10-yl)-1H-phenanthro[9,10-d]imidazole (3). The up-conversion of triplets to singlets via a triplet–triplet annihilation (TTA) process is dominant in these compounds due to 2ET1 > ES1. The pyrenyl dihydrobenzodioxin phenanthroimidazole (3)-based nondoped OLED exhibits blue emission (450 nm) with CIE (0.15, 0.14), a luminance of 53 890 cd m−2, power efficiency of 5.86 lm W−1, external quantum efficiency of 5.30%, and current efficiency of 6.90 cd A−1. The efficient device performance of pyrenyl dihydrobenzodioxin phenanthroimidazole is due to the TTA contribution to the electroluminescent process.


 Please wait while we load your content...
Please wait while we load your content...