Synthesis and evaluation of new sterol derivatives as potential antitumor agents†
Abstract
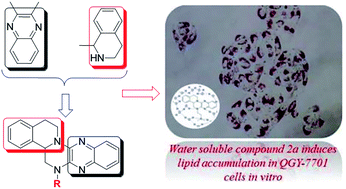
The current optimization of tetrazanbigen (TNBG) on the C-ring provided a series of new sterol derivatives 2a–2n. All new synthesized compounds were screened for their anti-proliferation activities against five human cancer cell lines (HepG2, QGY-7701, SMMC-7721, A-431 and NCI-H23 cell lines) in vitro. Among them, 2a, 2b, 2c, 2m and 2n exhibited high anti-proliferation activities on SMMC-7721, and their IC50 values approach that of the positive control drug cisplatin. Compound 2a not only showed strong anti-proliferation activities against QGY-7701 and HepG2 cell lines, with IC50 values (IC50: 6.81 ± 0.24 μM, 7.69 ± 0.87 μM) better than that of cisplatin (IC50: 8.75 μM, 18.89 ± 2.01 μM), but also exhibited good aqueous solubility (0.15–15 mg mL−1 at pH 7.4 and 2.0). On the most sensitive QGY-7701 cell line, Oil red O staining and western blot analysis were performed. The results suggested that 2a can inhibit the growth of cancer cells possibly by interfering with the lipid metabolism balance of tumor cells, resulting in lipid accumulation and cell apoptosis (lipotoxicity). Moreover, after being treated with 2a, lipid accumulation of QGY-7701 cell was increased in a time and dose dependent manner. Based on these promising results, 2a was selected for drug formulation and further pre-clinical development.


 Please wait while we load your content...
Please wait while we load your content...