An optimized procedure for direct access to 1H-indazole-3-carboxaldehyde derivatives by nitrosation of indoles†
Abstract
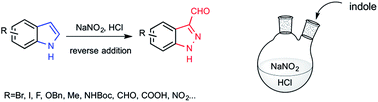
Indazole derivatives are currently drawing more and more attention in medicinal chemistry as kinase inhibitors. 1H-indazole-3-carboxaldehydes are key intermediates to access to a variety of polyfunctionalized 3-substituted indazoles. We report here a general access to this motif, based on the nitrosation of indoles in a slightly acidic environment. These very mild conditions allow the conversion of both electron-rich and electron-deficient indoles into 1H-indazole-3-carboxaldehydes.


 Please wait while we load your content...
Please wait while we load your content...