Organocatalytic asymmetric synthesis of tetrahydrocarbazoles via an inverse-electron-demand Diels–Alder reaction of 2,3-indole-dienes with enals†
Abstract
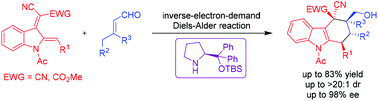
A novel 2,3-indole-diene synthon was designed and synthesized, and was first applied to a stereoselective inverse-electron-demand Diels–Alder reaction to construct tetrahydrocarbazoles. In the presence of chiral secondary amines, a variety of enantioenriched, multifunctionalized tetrahydrocarbazole derivatives were obtained in good yields (up to 83%) with excellent stereoselectivities (up to >20 : 1 dr, 98% ee).


 Please wait while we load your content...
Please wait while we load your content...