Tunable ESIPT reaction and antioxidant activities of 3-hydroxyflavone and its derivatives by altering atomic electronegativity
Abstract
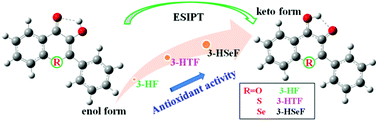
The effects of atomic electronegativity (O, S and Se atoms) on the excited-state intramolecular proton transfer (ESIPT) properties of three compounds (3-HF, 3-HTF and 3-HSeF) were systematically studied using the density functional theory (DFT) and time-dependent DFT (TD-DFT) methods. Furthermore, the antioxidant activities of the three compounds were evaluated using different parameters from the perspective of biological activities. The calculated results indicate that the generation of ESIPT is attributed to the excited-state intramolecular charge transfer owing to the change in electron density distribution on the frontier molecular orbitals and natural bond orbital (NBO) charge distribution of the O1 and O2 atoms at the S0 and S1 states. Moreover, 3-HTF shows the greatest red shift of the O1–H1 stretching peak and the lowest energy barrier at the S1 state among the three compounds, implying that 3-HTF would have the strongest intramolecular hydrogen bond at the S1 state, which is most beneficial for the occurrence of ESIPT. In addition, the decreased ionization potentials and energy gaps from 3-HF to 3-HTF and 3-HSeF means that the antioxidant activity of the compound would be enhanced along with the lowered atomic electronegativity. Interestingly, the lower ionization potentials and energy gaps of the compounds in keto forms suggest that the compound (3-HTF) easy to carry out ESIPT reaction would exhibit efficient antioxidant activity, which would establish the relationship between ESIPT reaction and antioxidant activity of the compound and provide a new notion for synthesizing more efficient antioxidants in experiments.


 Please wait while we load your content...
Please wait while we load your content...