Oxidative ring-opening of 3-aminoindazoles for the synthesis of 2-aminobenzoates†
Abstract
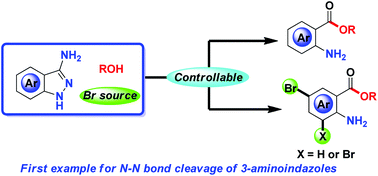
The first oxidative ring-opening of 3-aminoindazoles based on N–N bond cleavage is reported herein. A variety of 2-aminobenzoates were obtained in good yields under mild conditions, in which the free, mono- and dual-brominated aminobenzoates could be controllably achieved by employing the appropriate oxidant and bromine source.


 Please wait while we load your content...
Please wait while we load your content...