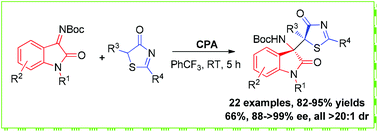
Organocatalytic enantioselective Mannich-type addition of 5H-thiazol-4-ones to isatin-derived imines: access to 3-substituted 3-amino-2-oxindoles featured by vicinal sulfur-containing tetrasubstituted stereocenters†
Abstract
The first chiral phosphoric acid catalyzed enantioselective nucleophilic addition of 5H-thiazol-4-ones to isatin-derived imines has been established. By using this strategy, the resulting 3-substituted 3-amino-2-oxindoles featuring both 5H-thiazol-4-one and vicinal sulfur-containing tetrasubstituted stereocenter structural motifs were obtained in high yields with excellent enantioselectivities and diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...