Alkylation–peroxidation of α-carbonyl imines or ketones catalyzed by a copper salt via radical-mediated Csp3–H functionalization†
Abstract
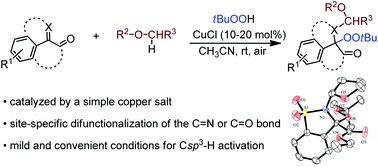
The catalytic alkylation–peroxidation of α-carbonyl imines or ketones was enabled by a simple copper salt under very mild reaction conditions. The transformations involved radical-mediated Csp3–H functionalization and afforded stable α-amino or α-alkyloxyl peroxides in good to excellent yields. This strategy would potentially provide a straightforward approach to direct difunctionalization of carbon−heteroatom double bonds and construction of structurally novel organic peroxides.


 Please wait while we load your content...
Please wait while we load your content...