Catalytic enantioselective and regioselective substitution of 2,3-indolyldimethanols with enaminones†
Abstract
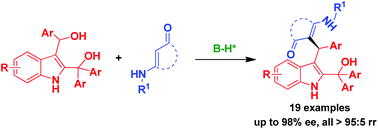
2,3-Indolyldimethanols as a new class of indolyldimethanols have been designed, and the first catalytic asymmetric substitution of 2,3-indolyldimethanols has been established by using cyclic enaminones as nucleophiles, which afforded substitutive products in an enantioselective and regioselective mode (up to 98% ee, all >95 : 5 rr). This reaction represents the first design and application of 2,3-indolyldimethanols in catalytic asymmetric reactions, which will greatly enrich the chemistry of indolyldimethanols.


 Please wait while we load your content...
Please wait while we load your content...