ipso-Arylative polymerization as a route to π-conjugated polymers: synthesis of poly(3-hexylthiophene)†
Abstract
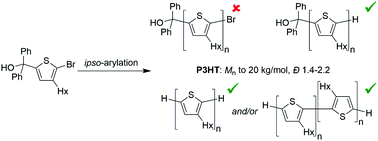
ipso-Arylative cross-coupling with two 3-hexylthiophene derivatives, (5-bromo-4-hexylthiophen-2-yl)diphenylmethanol and 2-(5-bromo-4-hexylthiophen-2-yl)propan-2-ol, has been used to prepare poly(3-hexylhiophene) (P3HT) as a model conjugated polymer. P3HT with number-average molecular weights ranging from 8–20 kg mol−1 (Đ 1.4–2.2) was prepared from 5-bromo-4-hexylthiophen-2-yl)diphenylmethanol with a Pd(OAc)2/PCy3/Cs2CO3 catalyst system. Only oligomerization of 2-(5-bromo-4-hexylthiophen-2-yl)propan-2-ol (Mn ≈ 3 kg mol−1) was observed under similar conditions. Studies with model compounds suggest that side reactions involving end-group loss limit ultimate molecular weights.
- This article is part of the themed collection: Frontiers in Supramolecular and Macromolecular Science symposia


 Please wait while we load your content...
Please wait while we load your content...